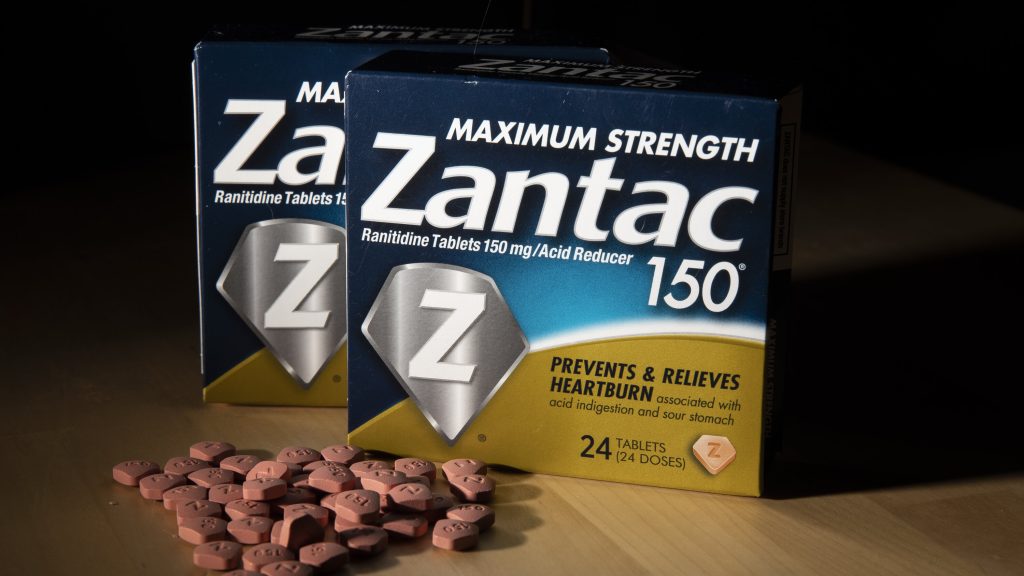
Zantac Lawsuit: Illinois Jury Rules in Favor of Boehringer Ingelheim.
An Illinois state jury has today ruled in favor of Boehringer Ingelheim, rejecting claims from two men who alleged that their prostate cancer diagnoses were caused by long-term use of the company’s over-the-counter heartburn medication, Zantac.
This verdict comes after two previous trials resulted in deadlocked juries, raising the stakes in the ongoing litigation surrounding the discontinued drug.
The legal battle has been complex, involving several major pharmaceutical companies and numerous plaintiffs who claim that the active ingredient in Zantac, ranitidine, breaks down into a carcinogen known as NDMA (N-Nitrosodimethylamine) when exposed to heat or over time.
Background: The Zantac Cancer Controversy
Zantac was once one of the most widely used heartburn medications, sold by companies such as Boehringer Ingelheim, Sanofi, Pfizer, and GSK. However, the U.S. Food and Drug Administration (FDA) requested in 2020 that manufacturers pull the drug off the market due to concerns over NDMA contamination, which is classified as a potential human carcinogen. The decision prompted a surge in lawsuits from consumers who claim that Zantac use contributed to their cancer diagnoses.
Over 70,000 lawsuits have been filed against drug manufacturers, alleging that ranitidine exposure led to various cancers, including prostate, stomach, and bladder cancers. These cases have been consolidated in various courts across the U.S., with plaintiffs accusing the drugmakers of negligence in failing to warn the public about the potential risks.
The Legal Landscape: Key Court Rulings and Challenges
The Illinois trial represents just one chapter in the ongoing legal saga surrounding Zantac. Boehringer Ingelheim was the sole defendant in this case after other companies, including GSK and Pfizer, had already reached settlements with plaintiffs. However, this trial ended with a verdict in favor of Boehringer Ingelheim, following two previous trials in Illinois that also ended in deadlocked juries.
In 2022, a major setback for plaintiffs occurred when a federal judge in Florida dismissed expert testimony for around 50,000 cases, ruling that the plaintiffs' expert witnesses lacked reliable scientific methods to prove the drug's carcinogenic effects. This ruling severely hindered the progress of the cases centralized in Florida, though some plaintiffs are continuing to appeal the decision.
The Delaware Supreme Court recently agreed to review a similar challenge, where drugmakers are seeking to exclude expert testimony in over 70,000 ongoing lawsuits in that state. This court ruling could have significant implications for the future of the remaining litigation.
Settlement Talks: Some Drugmakers Move Toward Resolution
Despite the legal hurdles, some pharmaceutical companies have begun to settle cases. Sanofi has reportedly agreed to settle around 4,000 lawsuits, while Pfizer has settled more than 10,000 cases. These settlements, however, may not mark the end of litigation for all affected consumers, as many lawsuits remain unresolved.
Plaintiffs’ legal teams are continuing to pursue cases in other states, despite setbacks in Florida and Illinois. The possibility of further settlements looms, though the outcome of ongoing trials will play a significant role in shaping the final resolution of the remaining lawsuits.
The Future of Zantac Lawsuits: Key Legal Considerations
The legal challenges surrounding Zantac are far from over, and several important legal angles remain to be explored in the coming months. One critical issue is whether plaintiffs can rely on expert testimony linking ranitidine to cancer, especially in the wake of federal court rulings that have limited the use of certain experts in these cases.
Additionally, the product liability aspect of the lawsuits raises questions about whether pharmaceutical companies can be held responsible for the public health consequences of selling a drug that allegedly contains dangerous levels of carcinogens. Plaintiffs' attorneys argue that the manufacturers were negligent in not properly warning consumers about the potential risks, while the defense maintains that the safety profile of Zantac was based on available scientific data at the time.
As litigation continues to unfold across the United States, affected individuals and legal professionals alike will be keeping a close eye on upcoming rulings. With more trials scheduled and settlements in motion, the full legal and financial impact of the Zantac lawsuits is yet to be determined.
Navigating the Zantac Legal Battles
The Zantac cancer lawsuits continue to be a significant focus in the pharmaceutical industry, raising complex legal questions surrounding product liability, consumer safety, and the validity of expert testimony. While recent rulings, including the Illinois verdict, have provided temporary wins for pharmaceutical companies like Boehringer Ingelheim, the larger battle over cancer claims related to Zantac is far from over. As settlements and court decisions shape the future of these cases, both consumers and drugmakers will likely face an evolving legal landscape in the coming months.
For now, plaintiffs and defendants alike will need to stay prepared as the ongoing litigation continues to develop in various jurisdictions.